top of page


Enhancing Patient Safety: The Significance of Post-Marketing Surveillance for Medical Devices
Medical devices have revolutionized healthcare, but they also come with inherent risks. Post-marketing surveillance (PMS) is a critical...
Regulatory Solutions India
Oct 17, 20232 min read


Medical Device Labelling: Ensuring Clarity and Safety
Medical device labelling is integral to patient safety and effective healthcare delivery. As per Chapter VI of India’s Medical Device...
Regulatory Solutions India
Sep 8, 20231 min read


Medical Device Grouping: Simplifying Regulation for Enhanced Efficiency
In the intricate realm of medical devices, robust regulations stand as sentinels of patient safety and product efficacy. Recognizing the...
Regulatory Solutions India
Aug 22, 20232 min read


CDSCO Medical Device Import License Renewal
The Medical Device Rule of 2017 stipulates that the medical device import license in India must be renewed every five years from the date...
Regulatory Solutions India
Jul 20, 20233 min read
How to Obtain CDSCO Medical Device Import License in India?
Introduction: The Indian healthcare sector is experiencing rapid growth, with the medical device market expected to reach $280 billion by...
Regulatory Solutions India
Jun 22, 20232 min read

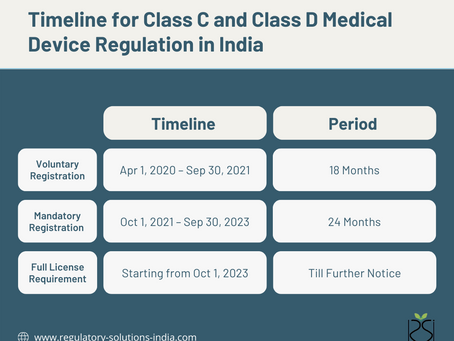
Full Licensing Requirement for Class C and D Medical Devices in India
Introduction: On April 12, 2023, the Central Drugs Standard Control Organization (CDSCO) of India issued a circular reminding Indian...
Regulatory Solutions India
Jun 15, 20233 min read


Understanding Post-Approval Changes in Medical Devices: A Comprehensive Guide
Introduction: Medical devices are subject to continuous change, which is an essential part of the development process. Sometimes, after...
Regulatory Solutions India
May 18, 20232 min read


New Medical Device Rules in India: Registration Certificate for Sale
Overview of the new amendments to the Medical Device Rule 2017 The Indian medical device industry is undergoing a major transformation...
Regulatory Solutions India
Apr 24, 20233 min read


Registration Process for Class A Non-Sterile and Non-Measuring Medical Devices in India
Medical devices are a vital part of the healthcare industry, playing a significant role in the prevention, diagnosis, treatment, and...
Regulatory Solutions India
Mar 17, 20232 min read

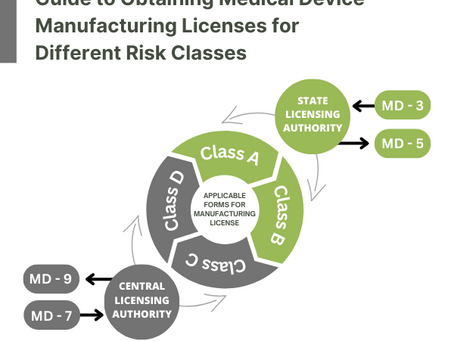
How do I get a manufacturing license for medical devices in India?
Obtaining a medical device manufacturing license in India is a complicated process that requires extensive documentation and compliance...
Regulatory Solutions India
Feb 24, 20232 min read


How do you register your medical device with CDSCO?
The Indian healthcare sector is expanding rapidly and is expected to reach $280 billion by 2025. India is considered one of the leading...
Regulatory Solutions India
Jan 20, 20233 min read
bottom of page
.png)